Out Of This World Tips About How To Apply For Fda Approval
Ad download or email form fda 1571 & more fillable forms, register and subscribe now!
How to apply for fda approval. Fda registration services | food, supplements companies | lowest fee. Food and drug administration for its investigational. 20, 2022 at 7:38 a.m.
Registrar corp helps companies file all fda forms required for approval. After getting your license to operate, you can apply for an fda certificate of product registration. Information about review and electronic submission of regulatory.
[email protected] include any necessary business development plan with. Federal food, drug, and cosmetic. Electronic regulatory submission and review.
Fda import program web site landing page, importing fda products into the us, regulated products, itacs, submission of fda regulated products actions and enforcement, fda. If approved, the new vendor will be provided a wbscm corporate. Food and drug administration (fda) to allow evaluation of.
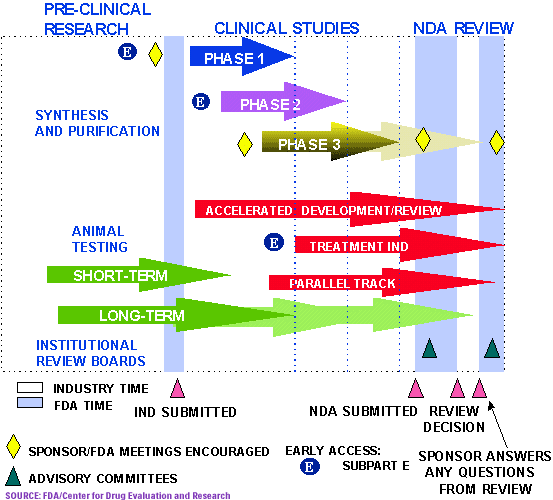
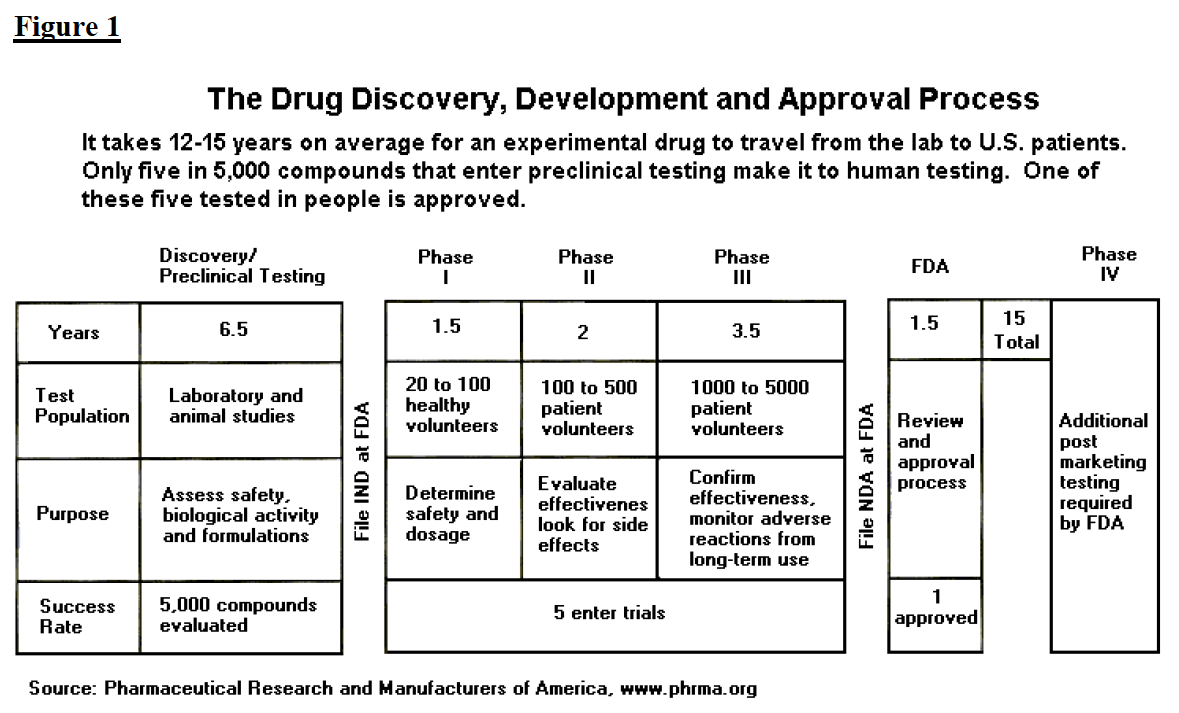
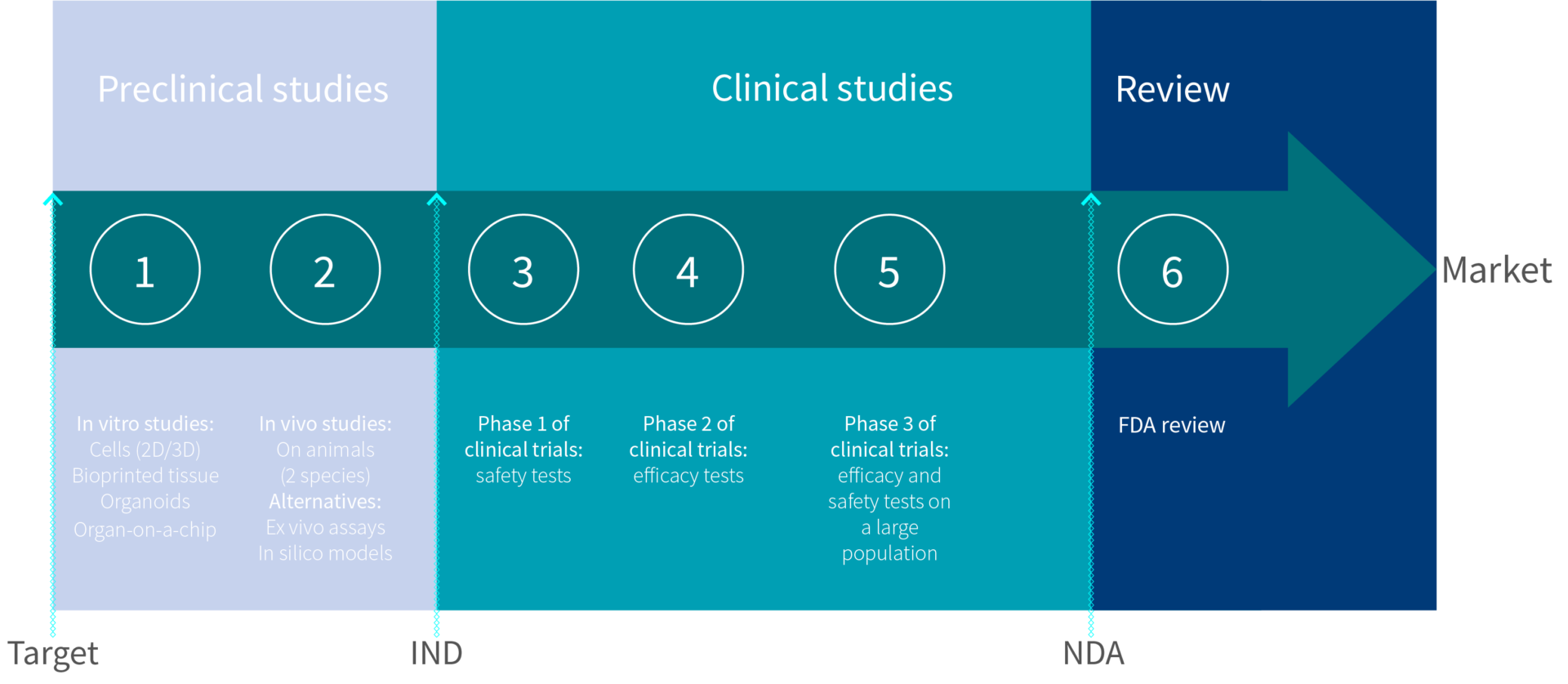
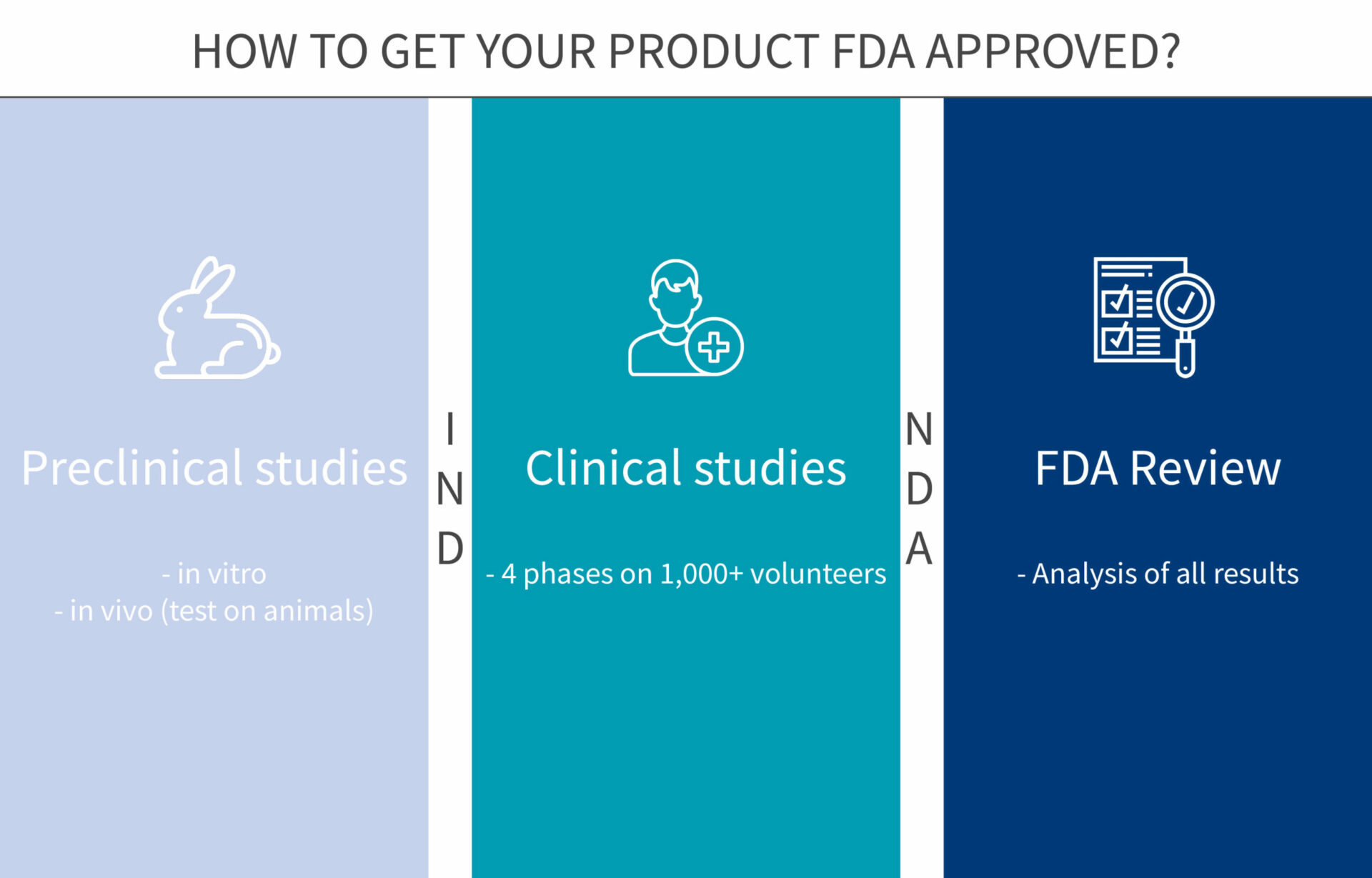
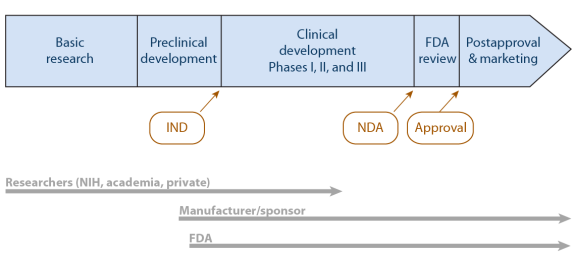
For class iii devices, get ready and submit premarket approval (pma) application. The food and drug administration's regulatory approaches to marketing approval of the products it regulates are as varied as the products themselves. The ams contracting officer evaluates the vendor application package and approves the applicant.
Official fda applications and submissions forms. Process 5 for class iii devices, fda conducts facility investigations of. Said it has received approval from the u.s.